Parkinson's disease – animal experiments and animal-free research
Parkinson's disease is a chronic progressive neurodegenerative disease. It induces typical motor symptoms such as stiffening of the muscles, slowed movements, and uncontrollable tremors. Other symptoms include sleep disturbances, loss of olfactory sense, and cognitive disorders. To this day, Parkinson's cannot be cured. The available medication only alleviates the symptoms and, at best, slows down the progression of the disease. Despite intensive - mainly animal-based - research, no significant improvement in therapy has been achieved in the last 40 years, and millions of patients and their relatives are waiting for help in vain. Modern animal-free research methods can achieve human-relevant results, increase the understanding of the human disease, and finally result in the development of an effective therapy.
The disease
Approximately 1% of the population over the age of 60 suffers from Parkinson's disease (1). Worldwide, these are about 6.2 million people, and it is estimated that this number will rise to over 12 million by 2040 (2).
The symptoms are based on the fact that cells in the so-called black matter (substantia nigra) in the midbrain of the patients perish. The affected nerve cells produce dopamine - a messenger molecule that nerve cells use to communicate with each other. As the dopamine-producing cells die, dopamine deficiency occurs, which leads to the typical motor symptoms of the disease (3). Deposits containing the protein α-synuclein, the so-called Lewy bodies, are found in the nerve cells of Parkinson's patients.
Although the disease has already been described over 200 years ago (1), its causes are still not understood. Several factors are suspected to be involved. In about 10% of cases, the disease is based on underlying genetic causes. In addition, the exposure to neurotoxins in particular is associated with the development of Parkinson's disease. Traumatic brain injury also increases the risk of the disease.
Current therapies
Many of the drugs used in the treatment of Parkinson’s aim to increase the concentration of dopamine in the brain or act on the dopamine receptors to restore the balance of neurotransmitters. The standard therapy is the dopamine precursor levodopa. However, the effectiveness of this therapy decreases over time. In addition, almost 50% of treated patients experience severe side effects such as movement disorders and psychosis within 5 years; with longer duration of treatment, the proportion of patients affected by these side effects increases even further (1). For patients who cannot achieve the desired improvement of their symptoms with medication, it is possible to have a so-called “brain pacemaker” implanted. It stimulates the nerve cells via electrodes which are permanently inserted into the brain (4). With this deep brain stimulation most patients can reduce their dose of medication and thus the corresponding side effects. Deep brain stimulation is also not a cure, and it does not work for all patients (1).
Recent studies investigated the use of stem cell transplantation, but they have only benefited younger patients and caused severe side effects. Moreover, the achieved "success" has been temporary (5). Gene therapies are also being studied for the treatment of Parkinson's disease but show little effect (1).
The disease and its causes are still not understood, and the effectiveness of available drugs and treatments, which are associated with significant side effects, is limited. Thus, there is still a considerable need for research.
Animal experiments in Parkinson's research
Parkinson's research uses so-called animal models, in which symptoms or changes reminiscent of Parkinson's are artificially induced in animals. Parkinson's-like symptoms are caused in a variety of ways, such as through administering drugs or toxins (poisons) or injecting them into the animals' brains (6). After such an "animal model" has been "produced", the success of the manipulation is monitored by movement and/or behavioural testing. A potential therapy is then carried out on the animals and the tests are repeated to determine whether there is an improvement in the artificially induced symptoms.
Drug-induced models
In the 1950s, it was observed that the psychiatric agents resperin and haloperidol can cause motor symptoms reminiscent of Parkinson's disease (7). This formed the basis for the development of the first so-called animal models for Parkinson's disease. Reperin blocks a membrane protein responsible for dopamine transport and thus leads to a reduction in dopamine concentration. However, it is not only the concentration of dopamine that is reduced, but also that of other monoamines, such as serotonin and norepinephrine, which are not affected in Parkinson's disease (6). In addition, the effect is not permanent (1). Resperin is injected into animals, mostly mice, but also other animals. The substance is often injected repeatedly in order to imitate the progression of the disease despite the limited duration of action. Haloperidol is used in another "animal model", causing a temporary catalepsy of the animals, which is a state of persistence in a rigid posture (6). The pharmacological models are easy to "produce", but only replicate partial aspects of the disease.
Toxin-induced models
In the 80s, it was discovered accidentally that the toxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) leads to Parkinson's-like symptoms after injection of a contaminated heroin substitute. Lipophilic MPTP can cross the blood-brain barrier and causes the death of dopamine-producing cells in the brain (8). MPTP leads to oxidative stress and mitochondrial dysfunction, which leads to inflammation and degeneration of dopamine-producing neurons in the black matter and thus to motor disorders. Other toxins used to cause Parkinson's-like symptoms in animals include pesticides such as rotenone, paraquat, and permethrin, as well as endotoxins derived from bacteria. While MPTP and the pesticides are administered systemically, endotoxins are injected directly into the brain, where they lead to the death of nerve cells.
Toxins lead to the rapid death of dopamine-producing nerve cells and thus to a fast development of movement disorders reminiscent of Parkinson's disease (6). However, this is an acute process and not a gradually progressive chronic neurodegenerative disease as it is in humans. Many aspects of the human disease are not reproduced, including the formation of the α-synuclein deposits, which are considered typical of Parkinson's disease (1).
Figure 1: Mice are injected with toxins that damage their nerve cells and thus lead to symptoms reminiscent of Parkinson's disease.
Genetic models
Approximately 10% of Parkinson's disease is based on genetics. With the discovery of various forms of familial, i.e., hereditary, Parkinson's disease, experiments began to reproduce the disease in "animal models" using genetic engineering. For this purpose, certain genes of the animals - especially mice are used here - are switched off, or human genes associated with the disease are incorporated into the genome of the animals.
One form of familial Parkinson's disease is characterized by a doubling or tripling of the gene encoding α-synuclein. This leads to an increase in α-synuclein expression and the formation of Lewy bodies, which are found in patients’ black matter but also in other brain regions. In addition, there are several gene mutations that occur in familial Parkinson's disease and are used to develop "animal models" (9)(1). Even if Parkinson's disease-associated mutations are caused in animals, they do not develop the clinical picture of Parkinson's. For example, almost all of these animal models lack the typical loss of dopamine-producing neurons, which is an essential part of the human disease (9).
α-synuclein-models
As described in the last section, the genetically determined overexpression of α-synuclein can be used to produce so-called animal models. In addition, there are other "animal models" that try to induce Parkinson's-like conditions in animals by increasing the α-synuclein concentration: The protein is injected directly into the animals' brains or viral vectors are used to cause the protein to be produced locally, i.e., directly in the animals' brains (6,9). Some of the "animal models" based on α-synuclein show behavioural changes, some show a reduction in dopamine concentration. However, in most cases, the manipulation does not lead to the death of the dopamine-producing neurons, so the condition of the animals is not comparable to Parkinson's disease.
Current state of animal-based Parkinson's research
None of the mentioned models are capable of replicating the complex human disease. Thus, the models supposedly have to be further optimized - in the belief that they can be designed to become more similar to the human disease. Meanwhile, the only thing the researchers can do is to investigate individual aspects of the complex disease in different "animal models" (6) and to combine different models with each other (9). In view of the generally poor translatability of the results from animal models (10) to humans, this concept – to investigate various aspects of a disease, which is not understood yet, in different animal models, whose transferability to humans is not given – does not seem promising.
Animal experiments in German Parkinson's research
The figures reported by the Federal Institute for Risk Assessment on the use of laboratory animals in Germany do not explicitly state the laboratory animals used for Parkinson's research. These figures are hidden in the categories of basic research on the subject of "nervous system", applied research on "human nervous and mental diseases", and "uses for regulatory purposes", in which, however, other diseases are summarized along with Parkinson's disease. In addition, animals that were bred for the production and maintenance of genetically modified lines – which are often used in Parkinson's research – as well as the so-called surplus animals, which were bred for Parkinson's research but not used, are not included in the numbers.
In order to gain an impression of the magnitude of the "animal consumption" of Parkinson's research in Germany despite of these difficulties, the AnimalTestInfo database can be used, in which the so-called non-technical project summaries (NTPs) of animal experiments approved in Germany are published (11). This does not result in the exact number of annual "consumption" of animals, since the NTPs, in the field of basic research for example, often contain rather vague justifications covering, for example, not only research into Parkinson's disease, but other neurodegenerative diseases such as Alzheimer's are also stated as the purpose of the experiments. Nevertheless, this approach makes it possible to at least approximate the number and type of laboratory animals that suffer in Germany in the name of Parkinson's research.
Searching the AnimalTestInfo database using the keyword "Parkinson's" for the year 2022 results in 61 animal experimentation projects. A total of 106,465 animals were approved. Of these, over 95% were mice. Thus, the mouse is the main victim in Parkinson's research; it was therefore also announced Laboratory Animal of the Year in 2019 by the Federal Association of People for Animal Rights (12).
In addition to mice, mainly rats are used in Parkinson's research. But zebrafish are also used in the hope that the regenerative capacity of their brains could provide clues for possible therapies for Parkinson's and other neurodegenerative diseases. In addition, the use of 47 macaques for regulatory purposes was approved. These animals are given drugs that are still under development or gene therapy to derive information on the toxicity and pharmacology of the substances.
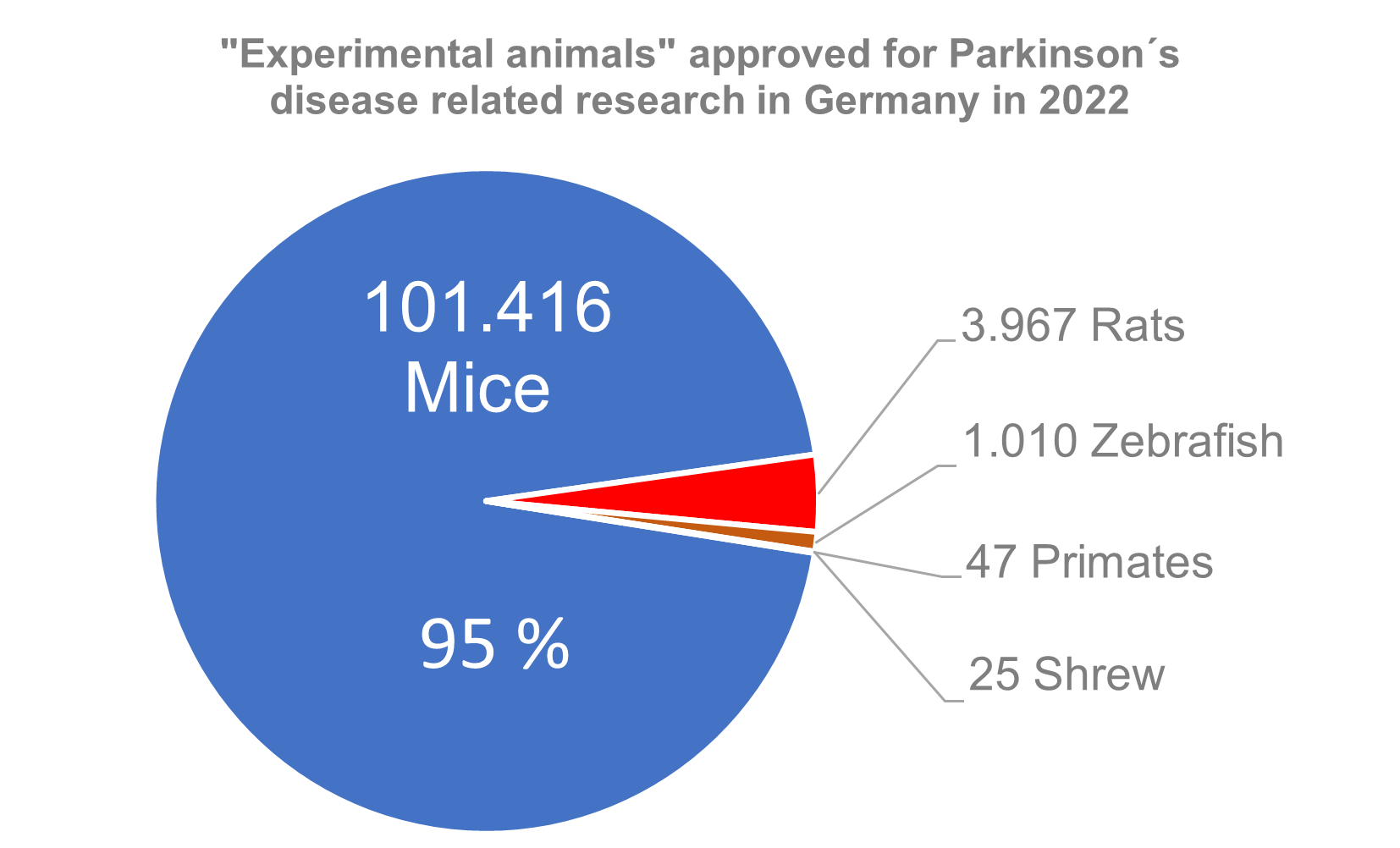
Figure 2: Estimation of animal “consumption” of German Parkinson's research.
Why animal experiments fail
For 60 years, more new animal models have been developed for Parkinson's research (1). Despite all these animal models, there has been no significant progress in the development of therapeutics since the 1950s. Available drugs so far only alleviate the symptoms and do not target the – still unknown – causes of the disease (1).
None of the animal models can actually simulate Parkinson's disease, each model only replicates individual aspects of the disease. Parkinson's is characterized by a slow perishing of nerve cells. Many "animal models" in Parkinson's research, on the other hand, are "produced" by a single, high-dose injection of a toxin. It is therefore not surprising that although 90% of the studies in mice, 95% of the studies in rats, and 67-80% of the studies in monkeys were successful, only 32% of the clinical studies in humans, which were based on the apparently successful preclinical studies using animals, brought clinical improvements (10,13).
And this is how Prof. Bernhard Hiebl from the University of Veterinary Medicine Hannover sums up the significance of animal experiments for Parkinson's and other neurodegenerative diseases: "There are more than 50 unsuccessful clinical studies on humans with drugs, all of which have previously been successful in animal models (14)".
Nevertheless, proponents of animal experiments still claim that the so-called animal models have been decisive for the success of Parkinson's research. For example, the MPTP model is said to have been crucial for the development of deep brain stimulation. In reality however, the method was investigated and developed through human studies decades before MPTP was administered to monkeys (4).
Animal-free Parkinson´s research
Due to the enormous limitations of the so-called animal models, better and human-relevant models are urgently needed to finally understand the disease and thus be able to develop possible therapies. These should focus directly on humans or even on individual patients.
Human and patient-derived cells
Since it is difficult to obtain primary human neurons and as they are also difficult to maintain in culture, immortalized human cell lines are often used in Parkinson's research. For example, the human neuroblastoma cell line SH-SY5Y is used (1). Even though research on these cells, has helped to conduct functional studies of some genes involved in Parkinson's disease, for example, the cells differ significantly from healthy nerve cells due to their origin from a tumour.
Stem cells can be differentiated into dopamine-producing nerve cells. Nowadays, one is no longer dependent on embryonic stem cells, but can e.g., transform skin cells into so-called induced pluripotent stem cells (iPSCs), which are able to differentiate into a large variety of cell types such as dopamine-producing neurons. The patient's own cells can be used, which then exhibit attributes characteristic of the respective patient. For example, neurons obtained from iPSCs from patients with a tripling of the α-synuclein encoding gene have increased concentrations of the protein (1). In addition to a better understanding of the disease, patient derived cells are particularly suitable for the development of new drugs. Many different potential drugs can be evaluated in parallel on the cells of different patients, so that drugs against different forms of Parkinson's can be found effectively.
Cell cultures can also be combined with microfluidic systems, which offer the possibility to precisely control the environment of the cells under investigation. For example, it is possible to set concentration gradients in microfluidics and thus investigate the dose-dependent effects of substances on neurons (15).
Organoid-based models
Organoids reflect the complexity of human tissue better than conventional two-dimensional cell cultures, and the use of three-dimensional cell culture models is particularly suitable in the context of the brain, where neurons are connected with each other. Brain organoids can be developed from the patient's own cells. They can contain dopamine-producing neurons and carry the genetic changes of the respective patient. Such organoids can be used to study the basis and mechanisms of the disease directly on human material (16). The influence of different mutations can be investigated by combining brain organoids with the so-called gene scissor CRISP/CAS, which can be used to insert altered genes or repair damaged genes. In addition to genetic Parkinson's disease, environmental factors can also be studied using organoids (17).
Organoids grown from human brain cells - if they are made from cells of patients - reflect their individual disease. They also offer the opportunity to investigate various forms of therapy (18).
Parkinson-on-a- chip-models
The combination of dopamine-producing neurons with other brain cells and endothelial cells of the blood vessels in a so-called organ-chip makes it possible to mimic Parkinson's on a chip. The blood-brain barrier, which must be overcome by Parkinson´s drugs, can be simulated on the chip. On such organ chips, it is possible to recreate various aspects of the disease. For example, the deposition of α-synuclein and the damage to the mitochondria as well as the inflammation of the nerve cells and the damage to the blood-brain barrier have already been observed and examined on a chip (19).
Parkinson´s-on-a-chip-models enable the inclusion of cell-cell interactions and the cellular environment and can be used for the testing of new drugs as well as for the study of disease development (20).
In-silico-models
Computer simulations, mathematical algorithms, and artificial intelligence enable the elucidation of molecular interactions. This can help predict both the efficacy and the side effects of a substance (1). These methods represent a valuable opportunity for the targeted development of new drugs, as they are based directly on the molecular basis of the disease (21).
Epidemiological studies
Parkinson's is not a uniform disease, but the courses of the disease vary between patients (22). A lot of relevant information can be obtained through population-wide or patient-based studies. This is the case, for example, with the Rotterdam study, which began in 1990. This is a prospective epidemiological study that has so far included three cohorts of several thousand participants each. The aim of the study is to investigate factors that determine the occurrence of various diseases in older people (23). Such epidemiological studies will make it possible to better understand the different forms of Parkinson's disease and to develop suitable therapies for the different subgroups of patients (10). Examination of brain tissue of deceased Parkinson's patients also provides crucial information. This is how the decades-long assumption that protein deposits in nerve cells are the cause of the disease, for example, has been refuted (24). This clearly shows that the search for the causes of the disease should be oriented towards the changes observed in humans.
Current status of animal-free Parkinson's research
Despite the development of animal-free research models and their advantages, Parkinson's research still focuses on its "animal models". An analysis of the literature revealed that the proportion of published research using human-relevant in-vitro models has remained constant over the past 40 years (10). This indicates an urgent need for a rethinking and a redistribution of research funds. This would not only reduce animal suffering but also accelerate developments in human-relevant Parkinson's research.
Prevention
Although the exact causes of Parkinson's disease are still unclear in most cases, there are certain risk factors that favour the disease. In addition to age and genetic predisposition, pollutants such as pesticides in particular represent a risk factor (6).
Already 40 years ago, it was observed that the toxin MPTP leads to the destruction of nerve cells in the black matter and the resulting characteristic Parkinson's symptoms (8). Since then, exposure to pesticides has also been found to increase the risk of Parkinson's disease (6,25). Thus, the handling of such pollutants as well as the pollution of our environment with them represent decisive risk factors. Here, the legislator has the duty to protect consumers from such substances in the best conceivable way.
Certain medications such as beta-adrenoceptor antagonists, better known as beta-blockers, are also associated with an increased risk for Parkinson's disease (26). Beta-blockers are often prescribed for hypertension. It should be evaluated whether the blood pressure can be normalized without such medication, for example by adjusting the patient’s lifestyle, by weight reduction, and an adapted, preferably plant-based diet.
Most population studies conducted show a link between the risk of developing Parkinson's disease and the consumption of milk and dairy products (26). The consumption of larger amounts of milk for example increases the risk of developing Parkinson's disease by more than a factor of 2 compared to people who do not drink milk (27). Avoiding dairy products can therefore make a significant contribution to reducing the personal risk.
Tobacco consumption appears to have a protective effect against Parkinson's disease, which may be due to the effect of nicotine on the central nervous system. However, it would also be possible for individuals who have a predisposition to Parkinson's disease to be less prone to smoking (26). However, as smokers have a drastically increased risk of an entire range of serious diseases, smoking is by no means advisable as a possible protection against Parkinson's disease.
Less risky is the consumption of tea or coffee, which reduces the risk of developing Parkinson's disease due to the neuroprotective effect of caffeine, theanine, and flavonoids.
Concluding remarks
The basics of Parkinson's disease are still not fully understood. In addition, it is a very variable disease, with different causes and different courses that respond differently to the different existing therapies. Nevertheless, Parkinson's research continues to focus on so-called animal models, in which individual aspects of the complex human disease are generated in young and genetically uniform animals. It remains questionable how it should be possible to simulate a human disease, which has not even been understood, in animals that cannot develop this disease naturally. These so-called animal models have nothing in common with the individual patients, most of whom develop Parkinson's disease at an older age and for distinct reasons. Consequently, it is not surprising that Parkinson's research has not created much progress for over 40 years. Non-animal methods, on the other hand, are used to conduct research directly on human material or even on patient-derived cells, which reflect the patient's individual disease. These methods, which already make a significant contribution to the research on Parkinson´s disease, must be promoted more strongly in order to develop their full potential. This would give justified reason for hope for patients who have so far waited in vain for a cure and improved therapies.
09 May 2023
Dr. Johanna Walter
Further Information
Parkinson's treatment with deep brain stimulation (brain pacemaker) >>
References
- Marshall L.J. et al. Parkinson’s disease research: adopting a more human perspective to accelerate advances. Drug Discovery Today 2018; 23(12):1950–1961
- Feigin V.L. et al. Global, regional, and national burden of neurological disorders, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. The Lancet Neurology 2019; 18(5):459–480
- Deutschen Gesellschaft für Parkinson und Bewegungsstörungen e.V.: Parkinson hat viele Gesichter.
- Ott C. et al. Parkinson-Behandlung mit Tiefenhirnstimulation (Hirnschrittmacher). 2017
- Freed C.R. et al. Transplantation of Embryonic Dopamine Neurons for Severe Parkinson’s Disease. New England Journal of Medicine 2001; 344(10):710–719
- Lama J. et al. Animal models of Parkinson’s disease: a guide to selecting the optimal model for your research. Neuronal Signaling 2021; 5(4):NS20210026
- Carlsson A. et al. 3,4-Dihydroxyphenylalanine and 5-Hydroxytryptophan as Reserpine Antagonists. Nature 1957; 180(4596):1200–1200
- Langston J.W. et al. Chronic Parkinsonism in Humans Due to a Product of Meperidine-Analog Synthesis. Science 1983; 219(4587):979–980
- Blesa J. et al. Challenges in Parkinson’s Disease, InTech, 2016
- Marshall L.J. et al. Poor Translatability of Biomedical Research Using Animals — A Narrative Review. Alternatives to Laboratory Animals 2023; doi: 10.1177/02611929231157756:026119292311577
- AnimalTestInfo
- Menschen für Tierrechte - Bundesverband der Tierversuchsgegner e.V.: Versuchstier des Jahres 2019: Die Maus in der Parkinson-Forschung
- Zeiss C.J. et al. Established patterns of animal study design undermine translation of disease-modifying therapies for Parkinson’s disease. PLOS ONE 2017; 12(2):e0171790
- Walsweer T.: Tierversuche: Geht’s auch ohne? VolkswagenStiftung 2019
- Seidi A. et al. A microfluidic-based neurotoxin concentration gradient for the generation of an in vitro model of Parkinson’s disease. Biomicrofluidics 2011; 5(2):022214
- Smits L.M. et al. Modeling Parkinson’s disease in midbrain-like organoids. npj Parkinson’s Disease 2019; 5(1):5
- Galet B. et al. Patient-Derived Midbrain Organoids to Explore the Molecular Basis of Parkinson’s Disease. Frontiers in Neurology 2020; 11:1005
- Smits L.M. et al. Midbrain Organoids: A New Tool to Investigate Parkinson’s Disease. Frontiers in Cell and Developmental Biology 2020; 8:359
- Pediaditakis I. et al. Modeling alpha-synuclein pathology in a human brain-chip to assess blood-brain barrier disruption. Nature Communications 2021; 12(1):5907
- Miccoli B. et al. Brain-on-a-chip Devices for Drug Screening and Disease Modeling Applications. Current Pharmaceutical Design 2019; 24(45):5419–5436
- Cruz-Vicente P. et al. Recent Developments in New Therapeutic Agents against Alzheimer and Parkinson Diseases: In-Silico Approaches. Molecules 2021; 26(8):2193
- Williams-Gray C.H. et al. The CamPaIGN study of Parkinson’s disease: 10-year outlook in an incident population-based cohort. Journal of Neurology, Neurosurgery & Psychiatry 2013; 84(11):1258–1264
- Ikram M.A. et al. The Rotterdam Study: 2018 update on objectives, design and main results. European Journal of Epidemiology 2017; 32(9):807–850
- Shahmoradian S.H. et al. Lewy pathology in Parkinson’s disease consists of crowded organelles and lipid membranes. Nature Neuroscience 2019; 22(7):1099–1109
- Hatcher J. et al. Parkinson’s disease and pesticides: a toxicological perspective. Trends in Pharmacological Sciences 2008; 29(6):322–329
- Belvisi D. et al. Modifiable risk and protective factors in disease development, progression and clinical subtypes of Parkinson’s disease: What do prospective studies suggest? Neurobiology of Disease 2020; 134:104671
- Park M. et al. Consumption of milk and calcium in midlife and the future risk of Parkinson disease. Neurology 2005; 64(6):1047–1051
