Cancer: Animal experiments and animal-free research
In the Western world, cancer is the second leading cause of death after cardiovascular diseases. 229,068 people died of cancer in Germany in 2021, representing 22.4% of all deceased (1). Despite decades of research on countless so-called animal models and vast amounts of invested time and research funds, the hope for a cure for cancer remains unfulfilled for many patients and their relatives. At the same time, new drugs are constantly entering the market, resulting in high profits for pharmaceutical companies, but often they have little benefits for the patients. This article sheds light on the reasons for the failure of cancer research, which is predominantly focused on animal experiments, and highlights human-relevant animal-free research methods.
Cancer
The term cancer refers to malignant deviations in various organs and blood cells. Cells get out of control, multiply in a rampant manner, become aggressive, and can penetrate and destroy the surrounding tissue. In Germany, 502,655 patients were diagnosed with cancer for the first time in 2019, according to estimates by the Centre for Cancer Registry Data. In about half of the cases, the mammary gland, prostate, colon, or lungs were affected (2). However, even if the same organ is affected by cancer, the tumor cells differ from patient to patient and even within a tumor the cells vary considerably (3). In addition, the tumor also changes over time and a therapy that initially works well by eliminating a portion of the cancer cells may become ineffective in the further course of treatment.
Treatment options
As diverse as different cancers and individual courses of the disease are, so are the treatment options: Tumors can be surgically removed, or radiation can be used to minimize tumor size or ideally eliminate the tumor completely. Classical chemotherapies slow down the division and multiplication of cancer cells. Anti-hormone therapies are intended to deprive certain tumors of their basis for growth. Novel cancer therapies are based on antibodies or immune cells that are designed to specifically target and kill cancer cells. Despite these diverse treatment strategies, which are often combined, the chance of survival for many cancers is still low and less than 20% for malignant tumors of the lungs, liver, and pancreas (2). Consequently, more and more anti-cancer drugs are being developed.
Development of new anti-cancer drugs
Between 2009 and 2013, the European Medicines Agency (EMA) approved 48 cancer drugs for 68 different indications. For only 24 indications (35%), a gain in survival time had been demonstrated in studies on patients at the time of approval. This gain in survival time averaged 2.7 months (1.0 – 5.8 months). A gain in quality of life was demonstrated for only 7 out of 68 indications (10%). After entering the market, the drugs were further evaluated and even after an average of 5.4 years of observation, an extension of survival time or an increase in quality of life was found for only 51% of the indications. Moreover, the gain of survival time for half of these indications was so small that it has no clinical significance (4).
In conclusion, many of the new drugs have a rather modest benefit. This is because criteria such as tumor size or short-term "stability" of the tumor are often used to evaluate new drugs. However, it is questionable whether these criteria result in a noticeable benefit for the patient. And even if the new drug is beneficial, the gain of a few months of life is often bought at the price of side effects, sometimes serious ones.
High failure rates in drug development
In the development of new cancer drugs, the attrition rate, i.e., the proportion of compounds that do not make it to the market, is particularly high. Only about 5% of the compounds for which an application for the approval of a new investigational drug (IND), which is necessary for the testing of new drugs in humans, has been submitted to the US Food and Drug Administration (FDA), reach the market (5). The main reasons why so many active substances fail are lack of efficacy and safety.
Why do so many compounds, whose efficacy and safety have been successfully demonstrated in the preclinical phase, fail in the clinical phase, when evaluated on humans? It is obvious that the methods used in preclinical research do not allow meaningful predictions about the effects of potential drugs on humans.
Cancer models
In cancer research, in addition to in vitro methods, such as cell cultures, so-called animal models are applied predominantly to investigate the causes and treatment options of the human disease (6). A wide variety of animal species are “used” to "produce" such models, most commonly small animals such as mice, rats, zebrafish, and fruit flies (6).
Mice are most commonly used, as they are cheap, easy to keep, and can be easily genetically modified (6). Zebrafish are also increasingly being used in cancer research, not based on their high similarity to humans and good prediction of the effect on the human organism, but because they are small, inexpensive, and can be bred quickly. Research is often conducted on zebrafish larvae, which are transparent, making it easy to observe, for example, the formation of metastases. Also, the genetic modification of zebrafish is comparatively simple, since the eggs are fertilized outside the body and do not have to be transferred to surrogate mothers. In this way, so-called zebrafish models can be established comparatively quickly for various diseases (6).
While smaller animal species are mainly used because of their simple reproduction, low cost, and ease of husbandry, some procedures such as surgery and radiation are easier to perform on larger animals. Therefore, pigs and dogs are also used in cancer research.
Animals in German cancer research
The "consumption" of animals in cancer research is enormous. In Germany, for example, 210,273 animals were used in 2021 in the fields of cancer-related basic research as well as in translational and applied research. It is not known how many additional animals were used for the breeding and maintenance of genetically modified animals, which is particularly common in cancer research. The number of animals used in the quality control of cancer drugs is also unknown. Together with animals bred for cancer research but killed as so-called "surplus animals" for several reasons such as the wrong age (7), the number of animals that actually suffer and die for cancer research in Germany is likely to be many times higher.
Table 1: Number of animals used for cancer research in Germany in 2021
|
|
Basic cancer research |
Applied Cancer Research |
Breeding of genetically modified animals |
Quality control |
Surplus animals |
|
Mice |
1023.46 |
104.900 |
? |
? |
? |
|
Rats |
454 |
727 |
? |
? |
? |
|
Guinea pigs |
10 |
- |
- |
? |
? |
|
Other rodents |
65 |
- |
- |
- |
? |
|
Cats |
4 |
- |
- |
- |
? |
|
Dogs |
- |
21 |
- |
- |
? |
|
Pigs |
45 |
120 |
- |
- |
? |
|
Xenopus |
30 |
- |
- |
- |
? |
|
Zebrafish |
1364 |
- |
? |
- |
? |
|
Other fish |
163 |
- |
- |
- |
? |
|
Rabbits |
10 |
9 |
? |
? |
? |
|
Rhesus monkeys |
- |
1 |
- |
- |
? |
|
Marmosets |
- |
4 |
- |
- |
? |
|
Total |
104.491 |
105.782 |
? |
? |
? |
How animals become "models"
Many cancers hardly occur naturally in animals. They have to be artificially induced in animals in order to "produce" so-called animal models for cancer research. Various methods are applied for this purpose.
- Chemical induction: Animals are treated with a carcinogenic substance. However, because it takes a long time for a tumor to form as a result, other "animal models" that are "ready for use" more rapidly are usually preferred.
- Genetic programming: In genetic programming, either certain cancer-promoting genes are activated or other genes normally preventing the formation of cancer, are switched off (6).
- Transplantation of cancer cells: Depending on the model, cancer cells of animal or human origin are injected into the animal and form a tumor at the injection site. This is often done under the skin of the animals, where tumor growth can be easily observed and measured. In other models, the tumor cells are injected directly into the organ where the original tumor formed. Animals with a defect immune system ("immunodeficient"), that cannot defend against the foreign cells, are often used for these models. As a result, the tumors grow at a rapid pace.
Due to the frequent use of "models" produced by transplantation of cancer cells, these are described in more detail in the following section.
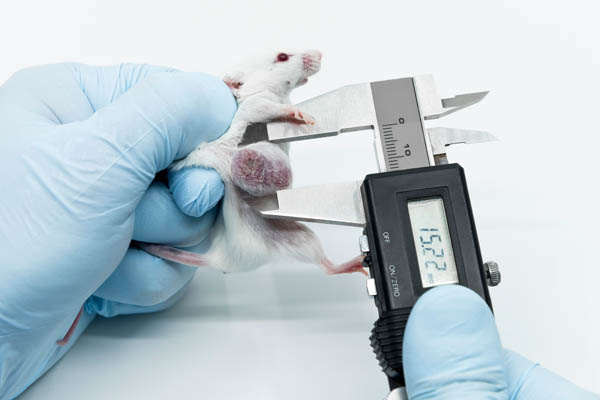
Xenograft models: Human tumor cells are often injected under the skin of mice because the tumor growth of subdermal tumors can be monitored easily.
CDX model
Cell lines derived from tumor tissue are often used to induce tumors. The corresponding "model" is called cell line-derived xenograft (CDX). However, the cell lines have been held in culture for countless generations and have thus adapted to the conditions under which they are propagated in the laboratory. As a result, the biological behavior of the cells has changed compared to the cells of the tumor from which they were originally obtained. In addition, a cell line cannot reflect the complexity of a tumor, which consists of different cells and changes over time.
PDX model
In the so-called PDX (patient-derived xenograft) model, samples obtained from biopsies or during the surgical removal of a tumor are used. The animal is injected with either cancer cells derived from that tumor or organoids cultured from these cells, or small pieces of the tumor itself are implanted. A human tumor then grows in the animal, which, at least initially, corresponds to the tumor of a particular patient. This method holds the potential to develop so-called personalized therapies for individual patients and has thus raised great hopes. However, not all tumor types are suitable for creating PDX models. In addition, the establishment of a PDX model often takes months - time that many patients do not have.
Limitations of CDX and PDX model
Even though in the PDX model the tumor growing in the animals is of human origin, it grows in mice, whose immune system differs significantly from that of humans. In addition, the mice are often genetically modified in such a way that they do not have a functioning immune system. Due to these discrepancies between the "model" and the human patient, the interactions between the immune system and the tumor cannot be replicated or predicted. In addition, the tumor grows in an environment that is vastly different from that in the human patient. The cells and structures surrounding it are of animal origin, and the signaling molecules with which the cells communicate with each other are different (8). Therefore, 60 – 70% of substances shown to be efficient in xenograft models cannot be transferred to humans (8) and most of the drugs successfully used in so-called animal models do not show the desired effects in human patients (6).
This has also been recognized by researchers. However, instead of questioning the models, in which animals are used as incubators for human tumors, new animal models are constantly being developed and “refined” in an attempt to deliver the long-awaited breakthrough in cancer research.
On the way to the perfect model?
In order to circumvent the problem that the tumor is confronted with a non-human and artificially damaged immune system, which reacts differently from the human immune system, numerous efforts have been made to reproduce partial aspects of the human immune system in animals.
For example, human hematopoietic stem cells, which are stem cells that are predominantly found in the bone marrow in humans and from which various blood cells, including immune cells, emerge, are implanted in mice. In other humanized models, cells from human embryos are implanted into the kidney capsule of immunodeficient mice, which are additionally injected with hematopoietic stem cells derived from the same embryo (9). In view of the limited availability of human stem cells and human embryos, and the regulation of the use of human embryos, human lymphocytes derived from donated blood are often injected into immunodeficient mice. This often leads to the so-called graft-versus-host disease (GvHD), in which the implanted human immune cells are fighting the cells of the mouse. As a result, the lifespan of these animals is reduced, which also shortens the possible duration of experiments (6).
Why animal experiments fail
Despite decades of research – not only on treatments for cancer, but above all on the development of more and more so-called animal models and the "refinement" of existing animal models – the yield of animal-based cancer research remains far behind the promises it has made. This is due to the poor transferability of the results obtained in animal experiments to humans, based on a number of reasons. The most obvious ones are the differences between different species. For example, it was found that only 46% of the chemicals that cause cancer in rats are also carcinogenic in mice (10). Since mice and rats are phylogenetically related much closer than mice and humans, it is hardly surprising that the development of human cancers cannot be understood in rodents.
In addition to the obvious differences between the different species, the entire branch of research also suffers from the desperate attempt to gain useful insights with obviously unsuitable models. "Animal models" are established on the basis of young healthy animals derived from genetically uniform inbred strains. These animals are then kept in the laboratory under standardized conditions. Often immunodeficient animals are used, i.e., they have been bred to have a defect immune system. These animals have nothing in common with the reality of cancer patients, who can differ significantly in age, genetic background, possible comorbidities, and lifestyle.
It is therefore not surprising that only about 5% of the compounds that reach the clinical phases are approved. Approximately 60% of the compounds fail due to lack of efficacy, although they achieved the desired anti-cancer effect in "animal models". This impressively demonstrates how the differences between animals and humans lead to the failure of cancer research.
Research directly on humans?
Since the differences between humans and animals are the main cause of the poor chances of success of novel anti-cancer drugs, it is obvious to think that direct research on humans would be more effective. And this is exactly what skeptics of animal experiments are often accused of. However, everyone should be aware that these experiments on humans are already routinely done under the more sonorous name "clinical study". In these studies, potential drugs - whose efficacy and safety were evaluated in preclinical studies by using inappropriate models and which can therefore be associated with significant risks - are evaluated in humans for the first time. Of course, new drugs should be as well studied and safe as possible before they are used in clinical trials. However, it is not at all conducive to the safety of participants in clinical trials if preclinical trials are conducted on animals whose organisms react very differently. Modern human-based model systems have already demonstrated to be better suited for evaluating the safety of active substances than so-called animal models (11).
Animal-free research methods
In preclinical research, i.e., in the development and testing of potential cancer therapies, cell-based methods are already being used in addition to animal experiments. Often, rather simple cell models are used for this purpose, such as human cancer cells, which either grow in a monolayer on cell culture surfaces or are examined as small mini-tumors in organoid format. These models are simple and inexpensive, and facilitate, for example, investigating whether a potential drug leads to the death of cancer cells or not. However, these models lack the necessary complexity because they are focused purely on the tumor cells and do not take into account the natural environment of the tumor, such as sprouting blood vessels or neighboring cells (12).
In order to circumvent these limitations, human-based models have been developed that also take into account the direct tumor environment and the systemic interaction of the potential drug with different organs.
Organoids and PDO models
Organoids are small round arrangements of cells. When they are obtained from tumor cells, they are more suitable to replicate certain properties of the tumor than simple monolayers of cells. For example, an oxygen and nutrient gradient forms towards the center of the organoid, as it also occurs in tumor tissue. If the tumor organoids are not obtained from cell lines but from biopsy material, they are referred to as patient-derived organoids (PDO), in which individual characteristics of the original tumor are preserved (13).
In contrast to the PDX model, PDO models can be obtained from any tumor type, are established in a few weeks, and enable high-throughput screening of drugs. PDO models are already being used today in the screening for new drugs (13). However, the PDO model focuses exclusively on the tumor and consequently the systemic effects of a substance cannot be determined.
Organ-on-a-chip models in cancer research
To take into account the interactions between tumor and other tissues as well as the metabolism, organ-on-a-chip systems have been developed. These are based on a microfluidic chip on which several small cultivation chambers are connected with each other by fine channels. In each of the chambers, different cells or tissues can be cultured and a fluid circulating through the channels mimics the bloodstream and transports nutrients and also potential drugs and their metabolites to the cells. Other aspects of the tumor environment can also be imitated, such as the extracellular matrix or natural barriers that can be reproduced by membranes overgrown with cells.
As an example, a microfluidic chip published by Jose M. Ayuso et al. in 2019 is outlined: In this system, tumor organoids are grown in a three-dimensional matrix that simulates the tumor microenvironment within the organism. Adjacent to this matrix are fine channels that are overgrown with endothelial cells and thus simulate blood vessels. Drugs and immune cells designed to fight the tumor can be introduced into these replicated blood vessels, allowing the system to simulate the systemic administration of drugs or immunotherapies. The model also enables the direct investigation of the interaction between tumor and immune cells (14).
In addition, the serial connection of different chambers in which cells from different organs are cultured enables not only the determination of the effect of a substance, but also the investigation of its pharmacokinetics, i.e., the distribution of the substance in different organs, and its conversion and degradation by different cell types.

Microfluidic systems enable the investigation of drugs in a human multi-organ system.
3D-printed tumor models
Tumors develop in a complex environment made up of different cells and the so-called extracellular matrix. This environment can be recreated using 3D printing. 3D bioprinting uses biocompatible "inks", for example hydrogels, in which cells are enclosed. In this way, different cell types, such as connective tissue, fat, and immune cells, as well as veins that supply the tumor with nutrients, can be printed in a defined three-dimensional arrangement (15). Other important bioactive components can also be introduced into the 3D construct. In this way, complex tissues, such as tumors, can be reproduced in vitro including their direct environment, which plays a vital role in the growth of the tumor and the formation of metastases, but also in whether and how well drugs can reach the tumor.
Of course, the mentioned in vitro methods can also be combined with each other. For example, tumor organoids can be integrated into 3D-printed constructs placed in a microfluidic system (16,17). In this way, the advantages of the individual systems can be used in synergy and tailor-made models can be made available for any scientific question.
Finding the underlying causes of cancer genesis
At the cellular level, cancer can develop based on changes in the genetic material (mutations). Most of these mutations occur spontaneously or are favored by external factors. Only about 5-10% of cancers are caused by pre-existing genetic defects (18). The other cases are caused by various environmental and lifestyle factors, many of which can be avoided or at least minimized.
The information available about how many of the cancer cases could be avoided varies. The WHO estimates that 30-50% of cancer cases worldwide could be prevented, the German Cancer Research Center (DKFZ) estimates that 37% of cancers in Germany could be avoided (2), and some experts assume that 60-70% of cancer cases are caused by tobacco and diet and are therefore preventable (18).
Prevention is possible
Up to 30-35% of cancer-related deaths can be attributed to diet, although the proportion can be much higher for certain types of cancer such as colorectal cancer (18). In addition to the intake of carcinogens such as nitrates, nitrosamines, and pesticides, this is mainly correlated to the consumption of meat, in which carcinogenic heterocyclic aromatic amines are produced during cooking or frying (19). On the other hand, a protective effect was found for plant-based foods (20).
25-30% of cancer-related deaths are caused by tobacco products. Tobacco contains over 50 carcinogens and the consumption of tobacco is responsible for 87% of deaths from lung cancer. In addition, tobacco use also increases the risk of at least 13 other cancers.
Another 15-20% of cancer-related deaths are caused by infections, mostly viruses. Viruses that can cause cancer include human papillomavirus (HPV), Eppstein-Barr virus, human immunodeficiency virus (HIV), and hepatitis B virus (HBV) (18). A vaccine for HPV has been available for several years to prevent the formation of cervical cancer. However, for many viruses including HPV the risk of infection can also be significantly reduced with comparatively simple means such as avoiding unprotected sexual intercourse.
The remaining cancer deaths can be attributed to other factors, such as radiation, stress, and pollution. Cancers are also promoted by alcohol consumption, obesity, and lack of exercise (21). For these cases, there are many possibilities for prevention too, from the simple application of sunscreen to measures that are much more difficult to implement but in many ways rewarding, such as the protection of our environment.
Conclusion: Conquering cancer with prevention and human-based research
While each individual can already make a significant contribution to his or her personal cancer prophylaxis through a healthy diet with a high proportion of plants and the abstinence from smoking, there are also factors that are harder or impossible to avoid. Consequently, and despite of all options for prevention, cancer research will continue to be necessary. However, in order for patients to benefit optimally from the research efforts, human-relevant models need to be used. This will facilitate to find the most effective and safe drugs on the one hand, but on the other hand will also prevent highly effective substances from being mistakenly sorted out because they fail or lead to unacceptable side effects in the wrong organism - in the animal.
What is the point of doing research in a complete model organism if it is the wrong organism that cannot predict the effect of a substance in the human body? And how much further would human cancer models be developed if all the ingenuity of researchers, all the time, and all the funding that currently goes into the development of new animal models would be invested into human-based research instead?
09 March 2023
Dr. Johanna Walter
References
- DESTATIS: Causes of death statistics 2021: 7% of all deaths are directly attributable to COVID-19 (in German), Press release No. 544, 16.12.2022, https://www.destatis.de/DE/Presse/Pressemitteilungen/2022/12/PD22_544_23211.html (accessed on 23.02.2023)
- Centre for Cancer Registry Data: Cancer total (in German), https://www.krebsdaten.de/Krebs/DE/Content/Krebsarten/Krebs_gesamt/krebs_gesamt_node.html (accessed on 23.02.2023)
- Sun X. et al. Intra-tumor heterogeneity of cancer cells and its implications for cancer treatment. Acta Pharmacologica Sinica 2015; 36(10):1219–1227
- Davis C. et al. Availability of evidence of benefits on overall survival and quality of life of cancer drugs approved by European Medicines Agency: retrospective cohort study of drug approvals 2009-13. BMJ 2017; doi: 10.1136/bmj.j4530:j4530
- Greek R. Animal models of cancer in light of evolutionary biology and complexity science. Americans For Medical Advancement, 2014.
- Li Z. et al. Application of animal models in cancer research: Recent progress and future prospects. Cancer Management and Research 2021; Volume 13:2455–2475
- Strittmatter S. 4 million animals killed as "surplus" in animal testing laboratories (in German). 2021
- Kumari R. Targeting the tumor microenvironment. GEN: Genetic Engineering & Biotechnology News 2014
- Lan P. et al. Reconstitution of a functional human immune system in immunodeficient mice through combined human fetal thymus/liver and CD34+ cell transplantation. Blood 2006; 108(2):487–492
- Di Carlo F.J. et al. Summary of carcinogenicity data generated by the national cancer institute/national toxicology program. Drug Metabolism Reviews 1984; 15(5–6):1251–1273
- Marshall L.J. et al. Poor translatability of biomedical research using animals - a narrative review. Alternatives to Laboratory Animals 2023; doi: 10.1177/02611929231157756:026119292311577
- Alves A.H. et al. The advances in glioblastoma on-a-chip for therapy approaches. Cancers 2022; 14(4):869
- Hou X. et al. Opportunities and challenges of patient-derived models in cancer research: patient-derived xenografts, patient-derived organoid and patient-derived cells. World Journal of Surgical Oncology 2022; 20(1):37
- Ayuso J.M. et al. Evaluating natural killer cell cytotoxicity against solid tumors using a microfluidic model. OncoImmunology 2019; 8(3):1553477
- Germain N. et al. Current advances in 3D bioprinting for cancer modeling and personalized medicine. International Journal of Molecular Sciences 2022; 23(7):3432
- Ma J. et al. Bioprinting of 3D tissues/organs combined with microfluidics. RSC Advances 2018; 8(39):21712–21727
- Wang X. et al. 3D bioprinted glioma microenvironment for glioma vascularization. Journal of Biomedical Materials Research Part A 2021; 109(6):915–925
- Anand P. et al. Cancer is a preventable disease that requires major lifestyle changes. Pharmaceutical Research 2008; 25(9):2097–2116
- Rohrmann S. et al. Heterocyclic aromatic amine intake increases colorectal adenoma risk: findings from a prospective European cohort study. The American Journal of Clinical Nutrition 2009; 89(5):1418–1424
- Vainio H. et al. Fruit and vegetables in cancer prevention. Nutrition and Cancer 2006; 54(1):111–142
- Report on the incidence of cancer in Germany 2016 (in German). Centre for Cancer Registry Data at the Robert Koch Institute. Berlin, 2016
