Endometriosis: Animal Experiments and Non-Animal Research
Endometriosis is one of the most common abdominal diseases in women. It is based on the formation of tissue similar to the lining of the uterus outside of the uterus. The disease is accompanied by considerable pain and a reduction in quality of life and can lead to involuntary childlessness. Endometriosis research, which is mainly based on animal experiments, has so far neither been able to elucidate the causes of the disease nor to enable a cure, and the effectiveness of existing therapies is limited. This article explains the background of these failures and shows how endometriosis research can succeed without animals.
The disease
In endometriosis, tissue reminiscent of the lining of the uterus, the so-called endometrium, is found outside of the uterus in the abdomen or also in more distant organs. The disease causes chronic abdominal pain as well as painful menstrual bleeding and often results in infertility. It can also lead to blood-filled cysts in the ovaries, called endometriomas. It is estimated that 2 to 20% of women have the condition, even if they do not show symptoms. As many as 40 to 60% of women who suffer from painful menstruation and 20 to 30% of women who have difficulties in getting pregnant are affected by endometriosis(1).
The causes of the disease are not yet fully understood. The theory of retrograde menstruation assumes that part of the menstrual blood does not drain to the outside but enters the abdominal cavity through the fallopian tubes. The blood contains endometrium cells, which can settle in the abdominal cavity and from which endometriosis foci can develop. However, there are also rare cases in which organs such as the lungs or the brain are affected by endometriosis, which cannot be explained by the theory of retrograde menstruation. Here, it is discussed whether endometriosis can arise in situ by cells of the corresponding organ differentiating into endometrium cells. This theory could also explain how, in rare cases, men or girls before puberty can develop the disease (2). Due to accumulation of endometriosis in some families, a genetic cause is also suspected (3).

Tissue similar to the lining of the uterus forms outside of the uterus.
Ref. Adobestock_Yatakviju
Treatment options
Analgesics, hormones, and surgery are treatment options currently available for endometriosis. Corpus luteum hormones (progestins) are used to suppress ovulation and menstruation. As a result, the endometriosis foci scattered in the body can become asymptomatic. However, this is no cure and after stopping the hormone treatment, the symptoms reappear. For women who have not finalized their family planning, long-term therapy with hormones is no option. In addition, hormones have a number of side effects such as weight gain, water retention, headaches, or mood swings. Treatment with drugs that inhibit the body's own production of female sex hormones is also used for endometriosis. However, this can lead to unpleasant menopausal symptoms.
The endometriosis lesions can be removed either surgically or obliterated with heat. In about 20% of women who have undergone surgery, endometriosis lesions recur within 5 years (4). Often, surgery is combined with drug therapy to avoid the formation of new foci. Removal of the uterus may lead to an improvement in symptoms, but it is not necessarily a cure. If the ovaries are removed at the same time, endometriosis is deprived of hormones. However, this also leads to menopause with corresponding changes of the body and well-being. If the symptoms of menopause are so severe that they are treated with hormones, endometriosis can also recur (4).
Development of new treatment options
While there are a number of new drugs currently in development, most of them target hormones. The few drugs with a different mechanism of action have not yet been fully studied (5).
Most drugs fail in phase II clinical trials, i.e. exactly when they are tested on patients for the first time (6). Since the safety of the substances reaching phase II has already been studied in phase I in healthy participants, this suggests that the subsequent failure in phase II is due to a lack of efficacy. The reason for this is thought to be the fact that the human disease and its development are poorly understood (6).
In summary, no novel therapeutic concepts have been developed in recent decades, which is mainly due to the poor understanding of the disease (5). This, in turn, is largely due to the animal experimental models used, which do not provide insights into the human disease.
The "animal models"
Since menstruation – at least according to the theory of retrograde menstruation – is a prerequisite for the spontaneous development of endometriosis, and the disease occurs almost exclusively in women during their fertile phase of life, the disease develops naturally only in humans and some non-human primates. Non-human primates have therefore been widely used to study endometriosis (7).
Primates in endometriosis research
Some non-human primates exhibit a menstrual cycle similar to that of humans. These animal species can spontaneously develop endometriosis. Spontaneous endometriosis has been reported in 11 monkey species, but rhesus monkeys and baboons are mainly used in research on the disease (1,7). The incidence of naturally occurring endometriosis in baboons is between 12 and 17% (8).
Since endometriosis develops slowly over a period of several years, methods have been developed to artificially induce the disease. Thus, in primates, menstrual flow is diverted to the abdomen by obstructing or closing the cervix to increase the amount of retrograde menstruation. In addition, surgical induction of the disease can be done by suturing fragments of endometrial tissue in ectopic (atypical) places or by seeding fragments of endometrial tissue into the abdominal cavity (7). However, the artificially induced endometriosis in primates is fundamentally different from the spontaneously developed disease in humans. The spontaneously developed disease also progresses differently in non-human primates when compared to humans, and there are hardly any more severe courses of the disease with an infestation of the ovaries in non-human primates (1).
In addition to ethical concerns about the use of primates, it is above all the high costs that prevent their widespread use in endometriosis research. For this reason, smaller laboratory animals, especially rodents, have been used to develop so-called animal models of endometriosis.
Rodents in endometriosis research
Bei Nagetieren kommt Endometriose nicht natürlich vor und muss daher künstlich hervorgerufen werden. Dazu wird Gebärmutterschleimhaut in die Bauchhöhle der Tiere implantiert. Je nachdem, ob dabei menschliches Gewebe oder solches aus der Maus verwendet wird, unterscheidet man zwischen heterologen und homologen Endometriose“modellen“.
Heterologous xenograft models
In the so-called heterologous xenograft model, human endometriosis tissue is implanted into the abdominal cavity or under the skin of rodents, such as mice. Since the immune system of the animals would recognize and attack the foreign tissue, immunodeficient mice are used for this purpose, which are usually genetically manipulated to exhibit a weakened immune system. In addition, the ovaries are removed from the animals and hormones are administered in an attempt to replicate the 28-day human menstruation cycle.
However, even in mice with a defective immune system, the human connective tissue cells and blood vessels are replaced by mouse cells within two weeks (5). Even when using animals with severe immunodeficiency, human tissue can only be preserved for a brief period of time, so that no long-term experiments are possible, and the chronic nature of endometriosis is not taken into account.
Due to genetic manipulation, so-called nude mice have a weakened immune system.
Homologous Models
In order to enable endometriosis research in immunocompetent mice, i.e., those with a functioning immune system, the endometrium from the same animal species or the same animal is used and implanted in different positions in the mouse. To do this, the uterus is removed from the animals and small pieces of it are reimplanted in the same animal, especially in the abdominal cavity or under the skin. Such models have been established for mice, rats, hamsters, and rabbits (7). In rats, fluid-filled cysts form from the implanted cells, which does not correspond to the situation in humans. Lesions in rabbits are also very different from those in humans (1). In addition, it has been shown that the artificial induction of endometriosis has no effect on fertility in mice, unlike in humans.
There are fundamental differences between the reproductive physiology of rodents and humans, resulting in significant limitations of rodent "models". Since most rodents do not menstruate, they do not develop spontaneous endometriosis, and the disease must be artificially induced by the transplantation of uterine tissue. In addition, in most of these rodent studies, the transplanted uterine fragments contain portions of the underlying muscles in addition to the uterine lining, which may affect the development of the lesions. Thus, the lesions induced in rodents do not sufficiently replicate those of humans. In addition, human endometriosis is a chronic disease that persists over extended periods of time and leads to scarring of the tissue. These alterations can lead to drugs not reaching the endometriosis foci at all – a circumstance that is not reflected in animal studies in which only artificially created and superficial endometriosis foci are examined (6).
Chicken embryos in endometriosis research
Chicken embryos are also used in endometriosis research. The eggshells are opened, and the underlying embryonic membrane is exposed. Fragments of human endometrial tissue are cultured on this membrane (4). Since the endometriosis lesions growing on the membrane can be easily observed, the model is primarily used to investigate the spreading of endometriosis and the involved formation of new blood vessels. The model is not suitable for the study of immunological or inflammatory components of the disease or for the study of active substances over a longer period of time.
Even though chicken embryos are substantially different from human patients, the model is used, among other things, because of the lack of legal protection for bird embryos. No authorization is required for experiments on chicken embryos in Germany, as Directive 2010/63/EU on the protection of animals used for scientific purposes applies only to mammalian embryos, but not to bird embryos (9). Consequently, experiments on chicken embryos are even presented by the experimenters as an "alternative" to animal experiments.
On the way to the perfect model?
The lack of predictive power of animal experiments results in researchers constantly revising and "refining" their models. This is difficult because endometriosis is a complex multifactorial and heterogeneous disease, the causes of which are still not yet elucidated Furthermore, the monitoring of so-called clinical endpoints such as pain or infertility is difficult and lengthy when using animal models. The researchers acknowledge the role of genomics and proteomics in endometriosis research, but instead of focusing on human studies, they expect that transgenic mice with human genes inserted into their genome will allow for better studies of the disease (7).
Why animal experiments fail
Endometriosis research, which is mainly based on animal experiments, has not yet produced a cure, and new treatment strategies - beyond intervening in the hormonal balance - are still lacking. The failure rate for the development of drugs in the field of women's health is over 95%. This means that more than 95% of active substances that have been tested to be effective and safe in animal experiments fail in the clinical phases - and thus in their first use in humans – mostly due to a lack of efficacy or safety (10). The main reason for this is the lack of translation, i.e., the lack of transferability of knowledge gained in animal experiments to humans. This is due on the one hand to the difference between humans and animals and, on the other hand, to the simplified and erroneous reproduction of endometriosis foci in the so-called animal models.
In addition, it is hardly possible to investigate the relevant clinical endpoints such as pain and reduced fertility in animal experiments. While researchers working with rodents and other "animal models" of endometriosis use reduction of lesion size and cell growth, or certain protein and gene expression data as alternative endpoints in their investigations, this has yielded only in sparse evidence for possible further developments in the treatment of human endometriosis (11).
The analysis of the available publications shows that animal experiments thus provide hardly any reliable data to provide drug candidates for clinical trials. As a result, even researchers themselves now doubt whether animal experiments are a suitable strategy for studying the pathophysiology of endometriosis and for developing new therapies (11).
Thus, there is an urgent need for new approaches that better reflect the human disease and have better predictive power (5).
Research directly on humans?
Important findings about endometriosis are already based on studies on humans. For example, samples were taken from endometriosis patients and patients without endometriosis and the gene expression of the tissues was compared. This has led to the identification of genes associated with the disease, providing important insights into the molecular basis of the disease (12). Clinical trials, observational and association studies also generate relevant results.
Clinical trials, observational and association studies also generate relevant results.
In order to provide the highest possible level of safety for the individuals involved in the clinical trials – and not to rely on such an undependable method as animal testing – the development and testing of new active substances should be carried out on systems derived from humans which provide human-relevant results.
Animal-free research methods
The study of endometriosis and corresponding drugs can be carried out directly with human cells, which is possible in various cultivation systems.
Cell cultures
The analysis of cells from endometriosis foci and of cell lines derived from them provides insights into the disease and enables the study of the contribution of different cell types. The comparison of endometriosis cells with those of healthy patients in particular provides insight into the biology of the disease. One problem, however, is that primary endometriosis cells rapidly dedifferentiate in cell culture and no longer represent the disease. This can be prevented, for example, by embedding the cells in a fibrin matrix (13). The use of the CRISPR-Cas genetic scissors makes it possible to generate precise changes in the genetic material of the cells, thus creating an opportunity to investigate the role of specific genes or mutations in endometriosis.
Organoids
Organoids are small 3D structures that can be developed in-vitro from healthy or diseased tissue, while retaining biological and pathological features of the original organ or disease. Endometriosis organoids thus represent promising models for investigating the molecular mechanisms of endometriosis. When endometrial organoids based on samples from healthy women and women with endometriosis are compared, a different gene expression pattern emerges, which provides information on the molecular basis of the disease (14). When the organoids are treated with progesterone, there is a dysregulation of gene expression in endometriosis organoids, which also explains why fertilized eggs cannot implant in the uterine lining of the respective women (14).
Endometrial cells for organoid development can even be obtained from menstrual blood (15). This represents a non-invasive way to obtain patient-specific organoids. In addition, the organoids can be cultivated, multiplied, and stored over a longer period of time and are therefore available in almost unlimited quantities. Organoids can also be embedded in hydrogels. When suitable hydrogels are used, organoid networks are formed by vascular cells (13).
Organ-on-a-Chip
Organ-on-a-chip systems are based on a so-called microfluidic chip, on which small cultivation chambers are connected to each other by fine channels. In each of the chambers, different cells or tissues can be cultured and a fluid circulating through the channels mimics the bloodstream and transports nutrients and hormones, or active ingredients to be tested to the cells. Microfluidic technology provides a controlled environment in which e.g., endometriosis organoids can be cultured under standardizable conditions. In addition, this technology enables precise control of cell-cell and cell-matrix interactions, as well as the reproduction of biochemical and mechanical signals and stimuli. The use of microfluidic systems also enables the integration of immune cells and other organs such as the liver or intestine (13), thereby recapitulating systemic aspects of endometriosis.
Co-cultivation of endometriosis organoids with lesion-derived stromal cells, as well as immune cells and muscle cells in synthetic extracellular matrices on microfluidic platforms can enable the replication of vascular lesions and their surroundings.
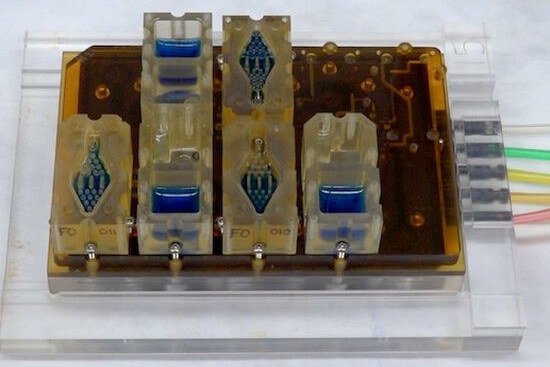
Female genitial tract on-a-chip.
Ref. Northwestern University.
3D Printed Models
Endometriosis lesions grow in a complex environment composed of different cells and the so-called extracellular matrix. This environment can be recreated using 3D printing. 3D bioprinting uses biocompatible "inks", for example hydrogels, in which cells are enclosed. In this way, different cell types such as endometriosis, muscle, and immune cells, as well as blood vessels, can be printed in a defined three-dimensional arrangement.
The various animal-free techniques can also be combined, for example by 3D printing of organoids in larger constructs or by using them in microfluidic systems. As a result, the in-vitro systems can be adapted and expanded as desired, allowing a wide variety of organs and functions to be imitated. This makes it possible to model and study ovulation, the menstrual cycle, and the implantation of an embryo in the endometrium in-vitro (16). Such complex systems, which consist only of human cells, will provide new insights into endometriosis research, and enable human-relevant testing of new drugs.
Investigating the causes of the disease
The causes of endometriosis are still unclear. The elucidation of the causes and underlying mechanisms of the disease, which is crucial for the development of therapies, can certainly not be achieved based on animal experiments in which only individual aspects of the disease are imitated. Population and patient studies combined with genome and proteome studies as well as human-based cell and tissue models are better suited to investigate the causes and mechanisms of endometriosis.
Prevention
The frequency of menstruation plays an essential role in the development of endometriosis. For example, pregnancy leads to an absence of menstruation and is a protective factor against endometriosis. The average number of pregnancies in Western Europe has decreased from about 10 to 1.5 in the last 100 years, which leads to a doubling of the menstrual frequency in a woman's life and thus also increases the risk of developing endometriosis (17). In order to artificially induce this absence of menstruation, long-term use of oral contraceptives is possible. This can reduce the recurrence of endometriosis after surgery. While such hormonal treatment may thus be useful for endometriosis patients under certain circumstances, it seems questionable whether it can contribute to the prevention of the disease (17). In view of the side effects of long-term use of hormones, this treatment cannot be recommended as a preventive measure.
However, women can influence their personal risk of endometriosis through their diet. In particular, there is a link between a pro-inflammatory diet and endometriosis (18). For example, the risk of developing endometriosis increases with the consumption of alcohol, unsaturated fatty acids, which are mainly found in dairy products, meat and highly processed foods, as well as with the consumption of red meat. On the other hand, the consumption of fruits, especially citrus fruits, reduces the risk of developing endometriosis by up to 22% (19). The symptoms of women with endometriosis can also be alleviated by adjusting their diet – especially by eating plenty of vegetables and fruits, as well as avoiding sugary drinks and animal fats (20).
Conclusion
Endometriosis research, which is mainly based on animal experiments, has not yet found a cure for the disease and the existing treatment options are unsatisfying. This is mainly due to the lack of predictive power of the so-called animal models, in which endometriosis-like conditions are artificially induced, but which do not reflect the complex human disease. This is even more problematic, as the causes of endometriosis have still not been identified, which means that the development of a causal treatment as well as a targeted prevention are still not possible today. Only the investigation of the disease in humans and the consistent use of human-relevant and animal-free research methods will be able to provide a remedy.
22.11.2023
Dr. Johanna Walter
References
- Story L. et al. Animal Studies in Endometriosis: A Review. ILAR Journal 2004; 45(2):132–138
- Sasson I.E. et al. Stem Cells and the Pathogenesis of Endometriosis. Annals of the New York Academy of Sciences 2008; 1127(1):106–115
- Rahmioglu N. et al. The genetic basis of endometriosis and comorbidity with other pain and inflammatory conditions. Nature Genetics 2023; 55(3):423–436
- Institut für Qualität und Wirtschaftlichkeit im Gesundheitswesen: Behandlungsmöglichkeiten bei Endometriose (abgerufen am 08.11.2023)
- Groothuis P. Model Systems in Endometriosis Research: Translation, Translation, Translation! Frontiers in Reproductive Health 2022; 3:809366
- Guo S.-W. et al. Is it time for a paradigm shift in drug research and development in endometriosis/adenomyosis? Human Reproduction Update 2018; 24(5):577–598
- Grümmer R. Animal models in endometriosis research. Human Reproduction Update 2006; 12(5):641–649
- D’hooghe T.M. et al. The prevalence of spontaneous endometriosis in the baboon (Papio anubis, Papio cynocephalus) increases with the duration of captivity. Acta Obstetricia et Gynecologica Scandinavica 1996; 75(2):98–101
- Richtlinie 2010/63/EU des Europäischen Parlaments und des Rates vom 22. September 2010 zum Schutz der für wissenschaftliche Zwecke verwendeten Tiere
- Kola I. et al. Can the pharmaceutical industry reduce attrition rates? Nature Reviews Drug Discovery 2004; 3(8):711–716
- Malvezzi H. et al. Endometriosis: current challenges in modeling a multifactorial disease of unknown etiology. Journal of Translational Medicine 2020; 18(1):311
- Esfandiari F. et al. HOX cluster and their cofactors showed an altered expression pattern in eutopic and ectopic endometriosis tissues. Reproductive Biology and Endocrinology 2021; 19(1):132
- Gnecco J.S. et al. Physiomimetic Models of Adenomyosis. Seminars in Reproductive Medicine 2020; 38(02/03):179–196
- Esfandiari F. et al. Disturbed progesterone signalling in an advanced preclinical model of endometriosis. Reproductive BioMedicine Online 2021; 43(1):139–147
- Cindrova-Davies T. et al. Menstrual flow as a non-invasive source of endometrial organoids. Communications Biology 2021; 4(1):651
- Mancini V. et al. Organs-On-Chip Models of the Female Reproductive System. Bioengineering 2019; 6(4):103
- Schweppe K.-W. Möglichkeiten zur Prävention der Endometriose – medikamentös und operativ. gynäkologische praxis 2019; 45(3)
- Liu P. et al. Association between dietary inflammatory index and risk of endometriosis: A population-based analysis. Frontiers in Nutrition 2023; 10:1077915
- Harris H.R. et al. Fruit and vegetable consumption and risk of endometriosis. Human Reproduction 2018; 33(4):715–727
- Ott J. et al. Endometriosis and nutrition – recommending a mediterranean diet decreases endometriosis-associated pain: an experimental observational study. Journal of Aging Research & Clinical Practice 2012; 1(2):162–166
